Normalized Data in Microbial Continuous Monitoring
Should I be concerned about normalizing data (CFU/m³) for grade A and B areas in Microbial Continuous Monitoring?

Filter:

Should I be concerned about normalizing data (CFU/m³) for grade A and B areas in Microbial Continuous Monitoring?

Typical ISO class cleanrooms in industries such as Pharmaceuticals or Semiconductors necessitate a standard set of demands for particle counting instrumentation and accessories to help customers achieve the required specifications outlined by regulatory committees. The Aerospace and Defense industries need to meet their own unique set of requirements that are often more variable and customer-specific than in other markets; this is something Particle Measuring Systems instrumentation and expertise can support.
Particle Measuring Systems has created these Cleanroom Standards, Compliance & Classification quick-reference cards. A free quick-reference tool designed to help you navigate cleanroom standards with ease.
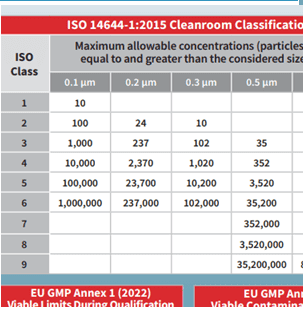
Simplify cleanroom compliance with these free, downloadable reference cards from Particle Measuring Systems. Designed for cleanroom operators and quality professionals, the cards summarize key ISO 14644-1 and EU GMP Annex 1 requirements, including particle limits, microbial guidelines, and cleanroom classifications.
Use them to stay compliant, support audits, and train your team.

This paper explores the application of a high-sensitivity liquid particle counter (Ultra DI® 20 Plus) to optimize ultrapure water (UPW) filtration performance in copper smelting and refining operations, and it demonstrates how sulfuric acid, a by-product of the process, can be refined to meet the high purity standards required by the semiconductor industry.

Discover the importance of compressed gas risk assessment in pharmaceutical manufacturing. Learn how to ensure product quality and compliance through effective management of compressed gas systems, including their design, operation, and monitoring.

Ensure the integrity of your manufacturing processes with our comprehensive guide on maintaining the cleanliness of compressed air systems. Discover essential guidelines for identifying contaminants, monitoring practices, and preventive maintenance strategies to meet regulatory standards. Learn how Particle Measuring Systems can support you in achieving optimal air purity and safeguarding product quality.
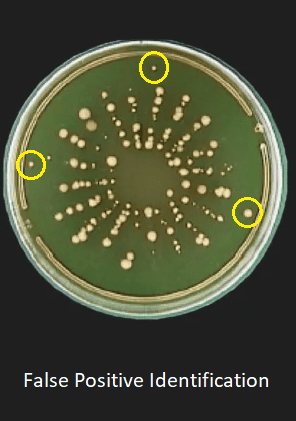
In this blog, we’ll explore how modern innovations, like the BioCapt® Single-Use microbial impactor, can enhance microbial air sampling efficiency while reducing the risk of false positives in cleanrooms.

Compressed gas systems play a critical role in pharmaceutical manufacturing and must be included in your Contamination Control Strategy (CCS). Annex 1 requires these systems to be properly designed, qualified, and monitored to prevent contamination.
👉 Read the full paper to learn how to assess compressed gas risk

In the pharmaceutical manufacturing industry, ensuring sterility and minimizing microbial contamination are critical for maintaining product quality and safety. Microbial sampling is a key component of environmental monitoring, and understanding the optimal criteria for sampling can significantly impact the effectiveness of sterility assurance programs. Learn about optimal microbial sampling criteria here.